Millions of people worldwide experience Raynaud’s phenomenon, yet for approximately 12.6% of these patients, those colour changes in their fingers may herald something far more serious—scleroderma, a potentially life-threatening connective tissue disease1. What appears as cold fingers turning white or blue might seem trivial, but distinguishing between primary (benign) and secondary (disease-associated) Raynaud’s phenomenon presents one of rheumatology’s most pressing diagnostic challenges.
The stakes are considerable. Primary Raynaud’s phenomenon generally remains harmless throughout a patient’s life, whereas secondary Raynaud’s phenomenon signals underlying systemic disease demanding immediate medical attention. Unfortunately, the diagnostic process frequently involves multiple tests and consultations, creating delays that can prove costly for patients who need urgent intervention.
Enter nailfold capillaroscopy—a safe, non-invasive diagnostic technique that has transformed how clinicians approach this differentiation. This method enables direct observation of structural changes in the microcirculation, the network of tiny blood vessels that nourish our tissues2. The technique’s diagnostic power is remarkable: nailfold capillary abnormalities appear in more than 95% of patients with systemic sclerosis1, while the characteristic scleroderma pattern demonstrates 89.47% sensitivity and 80% specificity for systemic sclerosis detection3.
This examination reveals how nailfold capillaroscopy functions as a diagnostic tool, the specific patterns it identifies across various connective tissue diseases, and its practical implementation in clinical practice. We’ll also explore how this technique integrates into the broader diagnostic framework and its essential role in monitoring disease progression over time. These insights empower clinicians to achieve more precise diagnoses, prevent misclassification, and ensure patients receive timely, appropriate care.
What Is Nailfold Capillaroscopy and Why It Matters
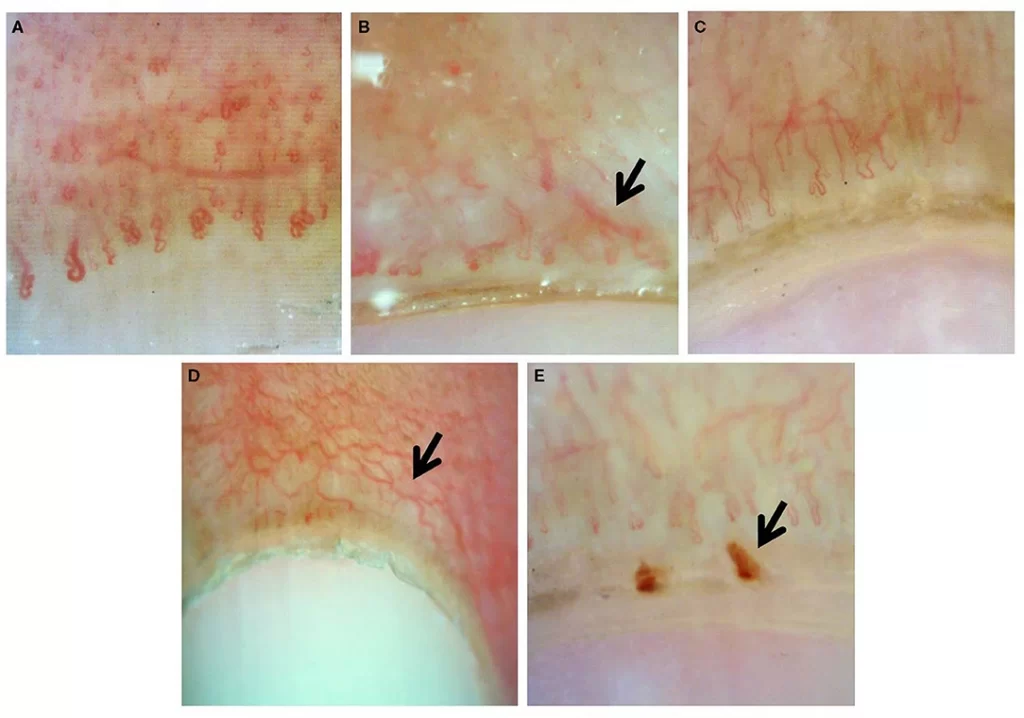
Image Source: Frontiers
Nailfold capillaroscopy functions as a highly sensitive, noninvasive imaging technique that evaluates microscopic blood vessels (capillaries) within the nailfold area4. This diagnostic method enables clinicians to directly observe and analyze the microvascular structure of capillaries running parallel to the skin surface at the nailfold, exposing their complete morphological characteristics5.
Visualizing Microvascular Changes in Real Time
The technique’s effectiveness stems from a unique anatomical advantage. Throughout most finger areas, capillaries orient perpendicular to the skin surface, rendering only their tips visible to examination. The nailfold region presents a different scenario—here, these tiny vessels run parallel to the skin surface, permitting complete visualization4. This anatomical arrangement creates an exceptional observation window for assessing microvascular health.
The examination process involves applying a drop of vegetable oil (such as walnut or olive oil) to enhance skin transparency and minimize surface reflection4. The resulting images expose critical quantitative parameters:
- Capillary density (normally 7-12 capillaries per linear millimeter)2
- Capillary dimensions (normal apical diameter around 20 μm)2
- Capillary morphology (shape variations from normal hairpin structure)2
- Presence or absence of microhemorrhages2
These parameters often show abnormalities months or years before clinical symptoms manifest in various rheumatic diseases, particularly systemic sclerosis spectrum disorders6. Early detection of these microvascular changes therefore enables therapeutic intervention before organ involvement develops.
Comparison with Other Imaging Techniques
Several optical instruments serve capillaroscopy purposes, each presenting distinct advantages and limitations.
Videocapillaroscopy combines microscopy with digital video camera technology, delivering magnification between 50× and 500×7. This technique represents the gold standard due to its superior image quality and measurement capabilities, yet its high cost and limited availability constrain widespread adoption. The technology permits sequential magnifications that enable detailed capillary assessment8.
Dermatoscopes provide portability and affordability despite offering lower magnification. A survey of systemic sclerosis specialists in the USA revealed that 64% utilize dermatoscopes or ophthalmoscopes, while only 7% employ videocapillaroscopy7. These devices function effectively as screening tools for distinguishing normal from abnormal patterns.
USB microscopy emerges as a promising alternative, delivering high-quality images at substantially reduced cost (available for as little as $25)9. These digital microscopes connect to computers via USB cables, facilitating panoramic capillary mosaics and automated measurements of vessel structure9.
EULAR Standardized Terminology for Capillary Features
The European League Against Rheumatism (EULAR) Study Group on Microcirculation in Rheumatic Diseases has developed standardized terminology for describing capillaroscopic findings10. This framework ensures consistent reporting across clinical settings and research studies.
EULAR consensus defines normal capillaries as hairpin, tortuous, or crossing structures11. Abnormal capillaries receive classification as “not hairpin, not tortuous, and not crossing”11. Image evaluation requires practitioners to assess:
- Capillary density (number per millimeter)
- Dimensions (particularly apical diameter)
- Morphological abnormalities (giant capillaries, ramifications)
- Presence of microhemorrhages6
This standardization gained particular importance following the inclusion of nailfold capillaroscopy in the 2013 ACR/EULAR classification criteria for systemic sclerosis12. Remarkably, even novice practitioners can reliably distinguish between normal and abnormal capillaries after just one hour of training, demonstrating the technique’s accessibility11.
The clinical significance of these standardized assessments extends beyond initial diagnosis to encompass disease progression monitoring and treatment response evaluation, particularly in conditions where microvascular damage drives pathogenic processes.
Capillaroscopic Patterns in Raynaud’s and Connective Tissue Diseases
Pattern recognition forms the cornerstone of capillaroscopic diagnosis. The microvascular changes visible through nailfold capillaroscopy create distinctive signatures that distinguish between primary and secondary Raynaud’s phenomenon—often appearing months or years before patients develop other symptoms of connective tissue disease.
Scleroderma Pattern: Early, Active, Late Stages
Among all capillaroscopic abnormalities, the scleroderma pattern stands out as the most thoroughly characterized and clinically significant. First described by Maricq in the 1980s, this pattern underwent refinement by Cutolo, who identified three distinct stages that mirror disease progression.
The early scleroderma pattern presents with:
- Few giant capillaries (≥50 μm in diameter)
- Few microhemorrhages
- Relatively preserved capillary distribution
- No significant capillary loss
Disease progression brings the active pattern, marked by:
- Frequent giant capillaries
- Frequent microhemorrhages
- Moderate capillary loss (20-30%)
- Mild disorganization of capillary architecture
- Absent or mild ramified capillaries
The late pattern represents advanced disease with:
- Severe capillary loss with extensive avascular areas
- Few or no giant capillaries
- Irregular enlargement of remaining capillaries
- Extensive ramified or bushy capillaries
- Severe disorganization of normal capillary array
This progression directly correlates with disease duration and severity. Patients with early-stage systemic sclerosis typically display the early pattern, while those with established disease show late-pattern changes. The evolution reflects the ongoing cycle of vascular damage and attempted repair that characterizes systemic sclerosis pathophysiology.
Capillary Changes in Dermatomyositis and Lupus
Other connective tissue diseases create their own recognizable capillaroscopic fingerprints. Dermatomyositis produces a “scleroderma-like pattern” with specific characteristics:
- Extremely enlarged capillaries and giant capillaries
- Extensive microhemorrhages
- Moderately ramified capillaries
- Capillary disorganization with “bushy” capillaries
These findings often correlate with disease activity and help clinicians differentiate dermatomyositis from polymyositis, which typically shows milder capillary changes.
Systemic lupus erythematosus (SLE) creates a “non-specific pattern” featuring:
- Tortuous capillaries
- Elongated loops
- Increased visibility of subpapillary venous plexus
- Occasional microhemorrhages without significant capillary loss
Unlike the relentless progression seen in scleroderma, microvascular changes in SLE fluctuate with disease activity. This temporal variability offers valuable insights into disease flares and remissions, helping clinicians adjust treatment strategies accordingly.
Mixed connective tissue disease exhibits a heterogeneous pattern that combines features from both scleroderma and lupus patterns, frequently displaying prominent capillary tortuosity alongside moderate capillary loss.
Sensitivity and Specificity of Capillaroscopic Findings
The diagnostic performance of nailfold capillaroscopy varies significantly across different conditions. For scleroderma, sensitivity ranges between 82-89.5% with specificity of 77-89.9%, establishing it as one of the most reliable non-invasive diagnostic tools available for this condition.
Giant capillaries alone demonstrate 84.2% sensitivity and 86.7% specificity for systemic sclerosis. Perhaps most importantly, capillaroscopy shows excellent negative predictive value—normal capillaroscopic findings virtually rule out systemic sclerosis in patients presenting with Raynaud’s phenomenon.
Dermatomyositis shows somewhat lower diagnostic accuracy, with sensitivity reaching approximately 60-70% and specificity around 80%. However, diagnostic value increases substantially when combined with other clinical parameters and serological markers.
Primary Raynaud’s phenomenon typically presents with normal capillaroscopic findings or minor non-specific abnormalities such as tortuous capillaries or mild dilatation. The absence of significant capillaroscopic abnormalities in a patient with Raynaud’s phenomenon strongly suggests a primary form, while the presence of a scleroderma pattern indicates secondary Raynaud’s with high probability.
Longitudinal studies reveal that 12.6-15% of patients initially diagnosed with primary Raynaud’s phenomenon who show capillaroscopic abnormalities will eventually develop definite connective tissue disease. This finding underscores the predictive value of this technique in identifying patients who require closer monitoring and follow-up care.
Clinical Workflow: When and How to Use Nailfold Capillaroscopy
Successful implementation of nailfold capillaroscopy demands structured protocols for patient selection, examination technique, and result interpretation. When properly executed, this diagnostic tool integrates seamlessly into both specialty rheumatology practices and primary care environments.
Indications for Capillaroscopy in Raynaud’s Phenomenon Investigation
Capillaroscopy has gained acceptance as a standard investigation tool among rheumatologists, particularly for differentiating primary from secondary Raynaud’s phenomenon13. The fundamental Raynaud’s evaluation should incorporate autoimmunity testing alongside nailfold capillaroscopy14. Clinical indications include:
- Persistent Raynaud’s symptoms with characteristic color changes triggered by cold or stress15
- Clinical suspicion of underlying connective tissue disease
- Ongoing monitoring requirements in established systemic sclerosis or related microvasculopathy conditions
The technique’s exceptional negative predictive value proves particularly valuable—normal capillary findings in Raynaud’s patients provide strong reassurance, while abnormal patterns indicate probable underlying systemic disease warranting additional investigation16.
Best Practices for Image Acquisition and Interpretation
Optimal examination requires adherence to specific protocols:
- Patient preparation: Allow 15-minute acclimatization to room temperature (20-22°C) before examination
- Application technique: Apply immersion oil drops to the periungual area for enhanced visualization
- Finger selection: Examine all fingers excluding thumbs, with optimal visibility typically at the fourth and fifth fingers of the non-dominant hand18
- Device positioning: Position handheld devices at 30-45 degrees between thumb and forefinger to minimize reflection
Systematic evaluation focuses on four essential parameters: capillary density (count per millimeter), capillary dimensions (especially width), morphological variations (shape abnormalities), and hemorrhage presence or absence19. Among quantitative measures, capillary density provides the most reliable indicator for predicting disease progression and monitoring treatment response19.
Using Dermatoscopes in Primary Care Settings
While videocapillaroscopy at 200× magnification remains the reference standard19, dermatoscopes present a practical alternative for primary care screening. Standard handheld dermatoscopes deliver approximately 10× magnification—adequate for initial assessment purposes20.
Dermatoscope examinations require approximately 4 minutes versus 18 minutes for standard microscopy21, making them suitable for busy clinical schedules. These devices are increasingly available in dermatology practices and primary care settings alike.
Practitioners should utilize the “MDAD approach” when performing dermatoscope examinations:
- Morphology: Evaluate capillary shape characteristics
- Diameter: Identify irregularly enlarged vessels
- Architecture: Assess organizational patterns
- Density: Count capillaries per millimeter18
General practitioners with basic dermatoscopy experience can effectively perform nailfold capillary examinations following brief training. Research demonstrates that even novice practitioners achieve reliable normal-versus-abnormal pattern differentiation after just 30 minutes of instruction22. This accessibility facilitates earlier secondary Raynaud’s recognition and appropriate specialist referral.
Predictive Value and Long-Term Monitoring
The diagnostic capabilities of nailfold capillaroscopy extend far beyond initial assessment. This technique provides substantial prognostic insights, enabling clinicians to monitor disease activity and anticipate organ complications in patients with Raynaud’s phenomenon and associated connective tissue diseases.
Capillary Loss as a Marker for Organ Involvement
Capillary density emerges as the most reliable predictor of clinical complications in systemic sclerosis. Multiple research studies confirm that progressive capillary loss correlates strongly with digital ulcer development, pulmonary arterial hypertension, interstitial lung disease, and overall disease severity23. A systematic literature review examining 18 studies found that 89% demonstrated positive associations between baseline capillaroscopic changes and clinical outcomes23.
Reduced capillary density independently predicts several serious complications: digital ulcer occurrence, progression of pulmonary vascular involvement, and worsening skin fibrosis23. The CAP study provided particularly compelling evidence, identifying that the mean number of capillaries per millimeter in the middle finger of the dominant hand served as one of three key predictors for new digital ulcer development within a 6-month period24.
Tracking Disease Progression in Systemic Sclerosis
Capillaroscopic patterns follow a predictable evolution throughout the disease course, progressing from early to active to late stages as microvascular damage accumulates. This evolution mirrors organ involvement severity directly2. Prospective studies reveal that progression from early to active pattern requires approximately 28±20 months, while progression from early to late pattern occurs within 36±29 months25.
Longitudinal observations of pattern changes reveal striking differences in progression rates. Patients whose microangiopathy advanced from early to late pattern showed markedly faster progression (11 months) compared to those progressing from early to active pattern (55 months)26. This accelerated progression was notably associated with nucleolar ANA pattern or Scl70 autoantibodies26.
Capillaroscopy in Risk Stratification and Follow-Up
Specialized prognostic indices based on capillaroscopic findings now guide clinical decision-making. The Risk Index of Ulceration in Systemic Sclerosis (CSURI) evaluates digital ulcer development risk within 3 months, achieving 92.9% sensitivity and 81.4% specificity25.
The Prognostic Index for Nailfold Capillaroscopic Examination (PRINCE) estimates the five-year probability of developing secondary Raynaud’s phenomenon in patients with isolated Raynaud’s27. When combined with autoantibody testing, prognostic accuracy improves significantly—patients with both abnormal capillaroscopy and SSc-specific antibodies demonstrate 80% likelihood of developing systemic sclerosis over 20 years27.
Conversely, normal capillaroscopic patterns provide exceptional reassurance. The technique demonstrates 97.1% negative predictive value for autoimmune disease development28, offering peace of mind for patients with Raynaud’s phenomenon who show normal nailfold capillaries.
Integrating Capillaroscopy into Patient Management
Successful management of Raynaud’s phenomenon demands a multifaceted approach that weaves together various diagnostic tools. Nailfold capillaroscopy forms the foundation of this assessment, yet its true clinical value emerges when paired with complementary investigations and patient-focused care strategies.
Combining Capillaroscopy with Blood Tests (ANA, ENA)
The diagnostic power of capillaroscopy multiplies when combined with serological testing. Primary care providers should routinely order antinuclear antibody (ANA) testing—a blood test that detects antibodies attacking the body’s own tissues—alongside capillaroscopy for patients presenting with Raynaud’s symptoms. When ANA screening proves positive, extractable nuclear antigen (ENA) profiling becomes essential to identify specific disease-associated antibodies29. This targeted approach detects:
- Anti-topoisomerase (Scl-70) antibodies in diffuse systemic sclerosis
- Anti-centromere antibodies in limited systemic sclerosis
- Anti-Ro/SSA or Anti-La/SSB in Sjögren’s syndrome
- Anti-dsDNA in lupus erythematosus30
The combined testing approach yields remarkable results. Patients with both abnormal capillaroscopy and specific autoantibodies face a 79.5% likelihood of developing systemic sclerosis and are 60 times more likely to develop the condition than those with neither predictor30.
Referral Guidelines for Suspected Secondary Raynaud’s
Certain clinical scenarios demand immediate rheumatology referral:
- Late-onset Raynaud’s (>30 years)—this timing typically signals secondary etiology31
- Positive ANA combined with puffy fingers31
- Digital ischemic lesions or ulceration31
- Abnormal capillaroscopy findings, regardless of blood test results32
Patients presenting with severe digital ischemia require urgent vascular surgery consultation31. For cases of suspected secondary Raynaud’s where diagnostic uncertainty persists, referral to specialized Raynaud’s clinics with advanced microvascular imaging capabilities offers the most appropriate next step31.
Patient Education and Lifestyle Modifications
Capillaroscopy serves a dual purpose beyond diagnosis—it becomes a powerful educational tool. Patients who view their own capillary images develop better understanding of their condition and demonstrate improved treatment adherence. Structural changes in nailfold capillaries correlate with modifiable lifestyle factors:
Lifestyle modifications guided by capillaroscopy findings can genuinely improve microcirculation. Research demonstrates that showing patients their abnormal capillaroscopic images prompted behavioral changes that resulted in improved capillary structure within 1-2 weeks17.
Patient education should address five key areas:
- Cold avoidance strategies
- Smoking cessation
- Stress management techniques
- Medication adherence
- Recognition of warning signs requiring urgent care
This article is for educational purposes only and does not constitute medical advice. Always consult a qualified healthcare provider for diagnosis and personalized care.
Conclusion
Nailfold capillaroscopy has established itself as a cornerstone diagnostic technique in the evaluation of Raynaud’s phenomenon. This simple examination of tiny blood vessels beneath the fingernails reveals critical information about microvascular health that often emerges months or years before patients develop obvious symptoms of serious autoimmune conditions.
The technique’s diagnostic strength lies in its ability to differentiate between harmless primary Raynaud’s and potentially dangerous secondary forms. Normal capillary patterns provide strong reassurance—they virtually exclude systemic sclerosis with 97% negative predictive value. Conversely, abnormal findings such as giant capillaries (blood vessels ≥50 μm in diameter), microhemorrhages (tiny bleeding spots), and capillary loss signal underlying connective tissue disease requiring immediate medical attention.
Capillaroscopy also functions as a powerful prognostic indicator. Reduced capillary density—the number of tiny blood vessels per millimeter—predicts which patients will develop digital ulcers, lung complications, and other organ involvement in systemic sclerosis. This predictive capability enables clinicians to identify high-risk individuals early, when therapeutic interventions prove most effective.
The technique’s accessibility represents another significant advantage. While specialized videocapillaroscopy equipment remains ideal, even basic dermatoscopes costing under $100 provide sufficient screening capability for primary care settings. This widespread availability facilitates earlier detection and appropriate specialist referral across diverse healthcare environments.
When combined with blood tests measuring autoantibodies (proteins that attack the body’s own tissues), capillaroscopy achieves remarkable diagnostic precision. Patients showing both abnormal capillary patterns and specific autoantibodies face 60 times higher risk of developing systemic sclerosis compared to those with normal findings.
The educational value of capillaroscopy extends beyond diagnosis. Patients who visualize their own capillary abnormalities demonstrate improved understanding of their condition and better adherence to treatment recommendations. This visual evidence motivates lifestyle changes that can improve microcirculation within weeks.
Capillaroscopy thus represents an essential tool in the modern evaluation of Raynaud’s phenomenon. Its integration into clinical practice reduces diagnostic uncertainty, prevents misclassification, and ensures patients receive appropriate care based on their individual risk profile rather than symptom severity alone.
Key Takeaways
Nailfold capillaroscopy transforms Raynaud’s diagnosis by revealing microscopic vascular changes that distinguish benign from serious underlying conditions.
• Capillaroscopy identifies secondary Raynaud’s with 89% sensitivity – abnormal patterns like giant capillaries and microhemorrhages signal underlying connective tissue diseases requiring immediate specialist referral.
- Normal capillary patterns virtually exclude systemic sclerosis – providing 97% negative predictive value, this reassures patients with primary Raynaud’s and avoids unnecessary investigations.
- Dermatoscopes make screening accessible in primary care – basic handheld devices costing under $100 enable effective screening after just 30 minutes of training.
- Combined testing maximizes diagnostic accuracy – pairing capillaroscopy with ANA/ENA blood tests increases systemic sclerosis detection likelihood by 60-fold compared to either test alone.
- Capillary density predicts organ complications – reduced capillary count strongly correlates with digital ulcers, lung disease, and overall disease severity in systemic sclerosis patients.
This non-invasive technique enables earlier detection of serious autoimmune diseases, allowing for prompt treatment before irreversible organ damage occurs. When integrated with blood tests and clinical assessment, capillaroscopy provides a comprehensive diagnostic approach that improves patient outcomes and reduces healthcare costs through targeted specialist referrals.
FAQs
Q1. What are the key lifestyle changes for managing Raynaud’s phenomenon? Avoid smoking and secondhand smoke, engage in regular exercise to improve circulation, practice stress management techniques, and minimize exposure to rapid temperature changes. These lifestyle modifications can help reduce the frequency and severity of Raynaud’s symptoms.
Q2. How does nailfold capillaroscopy help diagnose connective tissue diseases? Nailfold capillaroscopy allows visualization of microscopic blood vessels, revealing abnormalities like giant capillaries and microhemorrhages that are present in over 95% of systemic sclerosis patients. These changes often precede clinical symptoms, enabling early detection of underlying connective tissue diseases.
Q3. Is any special preparation required for a nailfold capillaroscopy test? No specific preparation is needed for nailfold capillaroscopy. The test is typically performed in a doctor’s office, and the healthcare provider will explain the procedure beforehand. Patients should simply arrive with clean, polish-free nails.
Q4. Can nailfold capillaroscopy distinguish between primary and secondary Raynaud’s phenomenon? Yes, nailfold capillaroscopy is highly effective in differentiating primary from secondary Raynaud’s. Primary Raynaud’s typically shows normal capillary patterns, while secondary Raynaud’s, associated with connective tissue diseases, displays distinctive abnormalities in capillary structure and organization.
Q5. How does combining capillaroscopy with blood tests improve diagnostic accuracy? Pairing nailfold capillaroscopy with serological tests like ANA and ENA significantly enhances diagnostic precision. Patients with both abnormal capillaroscopy findings and specific autoantibodies have a much higher likelihood of developing systemic sclerosis compared to those with normal results on either test alone.
References
[1] – https://www.mayoclinic.org/diseases-conditions/raynauds-disease/diagnosis-treatment/drc-20363572
[2] – https://www.sciencedirect.com/science/article/pii/S1521694223000359
[3] – https://pubmed.ncbi.nlm.nih.gov/24016612/
[4] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4569783/
[5] – https://pmc.ncbi.nlm.nih.gov/articles/PMC4918046/
[6] – https://link.springer.com/article/10.1007/s10067-019-04716-w
[7] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10341321/
[8] – https://www.rheumres.org/article_42084.html
[9] – https://academic.oup.com/rheumatology/article/60/8/3862/6000242
[10] – https://www.sciencedirect.com/science/article/abs/pii/S000349672456099X
[11] – https://academic.oup.com/rheumatology/article/55/5/883/1744697
[12] – https://www.sciencedirect.com/science/article/pii/S2255502114001904
[13] – https://www.sciencedirect.com/science/article/pii/S156899722030001X
[14] – https://reumatologiaclinica.org/en-clinical-impact-nailfold-capillaroscopy-in-articulo-S2173574320300411
[15] – https://aariarheumatology.com.sg/procedures/nailfold-capillaroscopy/
[16] – https://pmc.ncbi.nlm.nih.gov/articles/PMC10234192/
[17] – https://pmc.ncbi.nlm.nih.gov/articles/PMC9200324/
[18] – https://www.racgp.org.au/afp/2015/november/nailfold-dermatoscopy-in-general-practice
[19] – https://www.rheumatologyadvisor.com/features/recommendations-for-nailfold-capillaroscopy-in-assessment-of-raynaud-phenomenon-and-systemic-sclerosis/
[20] – https://www.the-rheumatologist.org/article/rheumatologists-find-nailfold-capillaroscopy-increasingly-useful-diagnostic-tool/5/?singlepage=1
[21] – https://jamanetwork.com/journals/jamadermatology/fullarticle/479453
[22] – https://academic.oup.com/rheumatology/article/62/7/2335/6795003
[23] – https://pmc.ncbi.nlm.nih.gov/articles/PMC8922564/
[24] – https://pmc.ncbi.nlm.nih.gov/articles/PMC5129545/
[25] – https://www.elsevier.es/es-revista-revista-colombiana-reumatologia-english-edition–474-articulo-prognostic-value-capillaroscopy-in-organ-S2444440520300492
[26] – https://pubmed.ncbi.nlm.nih.gov/31750929/
[27] – https://www.the-rheumatologist.org/article/rheumatologists-find-nailfold-capillaroscopy-increasingly-useful-diagnostic-tool/?singlepage=1
[28] – https://www.sciencedirect.com/science/article/abs/pii/S0003496724081019
[29] – https://rightdecisions.scot.nhs.uk/tam-treatments-and-medicines-nhs-highland/adult-therapeutic-guidelines/rheumatology/raynauds-phenomenon-guidelines/
[30] – https://pmc.ncbi.nlm.nih.gov/articles/PMC6139949/
[31] – https://remedy.bnssg.icb.nhs.uk/adults/rheumatology/raynauds-phenomenon/
[32] – https://www.portailvasculaire.fr/sites/default/files/docs/2017_esvm_raynaud.pdf